From Wikipedia, the free encyclopedia
| ||||||||||||||||||||||||||||||||||||||||
| General | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, symbol, number | chromium, Cr, 24 | |||||||||||||||||||||||||||||||||||||||
| Chemical series | transition metals | |||||||||||||||||||||||||||||||||||||||
| Group, period, block | 6, 4, d | |||||||||||||||||||||||||||||||||||||||
| Appearance | silvery metallic | |||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 51.9961(6) g·mol−1 | |||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d5 4s1 | |||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 13, 1 | |||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||
| Phase | solid | |||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 7.15 g·cm−3 | |||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 6.3 g·cm−3 | |||||||||||||||||||||||||||||||||||||||
| Melting point | 2180 K (1907 °C, 3465 °F) | |||||||||||||||||||||||||||||||||||||||
| Boiling point | 2944 K (2671 °C, 4840 °F) | |||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 21.0 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 339.5 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||
| Heat capacity | (25 °C) 23.35 J·mol−1·K−1 | |||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||
| Crystal structure | cubic body centered | |||||||||||||||||||||||||||||||||||||||
| Oxidation states | 6, 4, 3, 2 (strongly acidic oxide) | |||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.66 (Pauling scale) | |||||||||||||||||||||||||||||||||||||||
| Ionization energies (more) | 1st: 652.9 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||
| 2nd: 1590.6 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||
| 3rd: 2987 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 140 pm | |||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 166 pm | |||||||||||||||||||||||||||||||||||||||
| Covalent radius | 127 pm | |||||||||||||||||||||||||||||||||||||||
| Miscellaneous | ||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | AFM (rather: SDW) | |||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 125 nΩ·m | |||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 93.9 W·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 4.9 µm·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 5940 m/s | |||||||||||||||||||||||||||||||||||||||
| Young's modulus | 279 GPa | |||||||||||||||||||||||||||||||||||||||
| Shear modulus | 115 GPa | |||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 160 GPa | |||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.21 | |||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 8.5 | |||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 1060 MPa | |||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 1120 MPa | |||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-47-3 | |||||||||||||||||||||||||||||||||||||||
| Selected isotopes | ||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||
Chromium (IPA: /ˈkrəʊmiəm/) is a chemical element in the periodic table that has the symbol Cr and atomic number 24. It is a steel-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odourless, tasteless, and malleable.
[edit] History
On 26th July 1761, Johann Gottlob Lehmann found an orange-red mineral in the Ural Mountains which he named Siberian red lead. Though misidentified as a lead compound with selenium and iron components, the material was in fact lead chromate with a formula of PbCrO4, now known as the mineral crocoite.
In 1770, Peter Simon Pallas visited the same site as Lehmann and found a red "lead" mineral that had very useful properties as a pigment in paints. The use of Siberian red lead as a paint pigment developed rapidly. A bright yellow made from crocoite became a color in fashion.
In 1797, Louis Nicolas Vauquelin received samples of crocoite ore. He was able to produce chromium oxide with a chemical formula of CrO3, by mixing crocoite with hydrochloric acid. In 1798, Vauquelin discovered that he could isolate metallic chromium by heating the oxide in a charcoal oven. He was also able to detect traces of chromium in precious gemstones, such as ruby, or emerald. Later that year he successfully isolated chromium atoms.
During the 1800s chromium was primarily used as a component of paints and in tanning salts but now metal alloys account for 85% of the use of chromium. The remainder is used in the chemical industry and refractory and foundry industries.
Chromium was named after the Greek word "chroma" meaning color, because of the many colorful compounds made from it.
[edit] Occurrence and production
Chromium is mined as chromite (FeCr2O4) ore. About two-fifths of the chromite ores and concentrates in the world are produced in South Africa. Kazakhstan, India, Russia and Turkey are also substantial producers. Untapped chromite deposits are plentiful, but geographically concentrated in Kazakhstan and southern Africa.
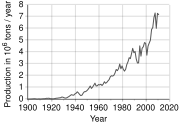
Approximately 15 million tons of marketable chromite ore were produced in 2000, and converted into approximately 4 million tons of ferro-chrome with an approximate market value of 2.5 billion United States dollars.
Though native chromium deposits are rare, some native chromium metal has been discovered. The Udachnaya Mine in Russia produces samples of the native metal. This mine is a kimberlite pipe rich in diamonds, and the reducing environment so provided helped produce both elemental chromium and diamond. (See also chromium minerals)
Chromium is obtained commercially by heating the ore in the presence of aluminium or silicon.
[edit] Chemical properties
Chromium is a member of the transition metals, in group 6. Chromium(0) has an electronic configuration of 4s13d5, due to the lower energy of the high spin configuration. Chromium exhibits a wide range of possible oxidation states. The most common oxidation states of chromium are +2, +3, and +6, with +3 being the most stable. +1, +4 and +5 are rare. Chromium compounds of oxidation state +6 are powerful oxidants.
Chromium is passivated by oxygen, forming a thin protective oxide surface layer which prevents oxidation of the underlying metal. This makes chromium suitable for a plating material.
[edit] Compounds
See also chromium compounds. Potassium dichromate is a powerful oxidizing agent and is the preferred compound for cleaning laboratory glassware of any trace organics. It is used as a saturated solution in concentrated sulfuric acid for washing the apparatus. For this purpose, however, sodium dichromate is sometimes used because of its higher solubility (5 g/100 ml vs. 20 g/100 ml respectively). Chrome green is the green oxide of chromium, Cr2O3, used in enamel painting, and glass staining. Chrome yellow is a brilliant yellow pigment, PbCrO4, used by painters.
Chromic acid has the hypothetical structure H2CrO4. Neither chromic nor dichromic acid is found in nature, but their anions are found in a variety of compounds. Chromium trioxide, CrO3, the acid anhydride of chromic acid, is sold industrially as "chromic acid".
[edit] Chromium and the quintuple bond
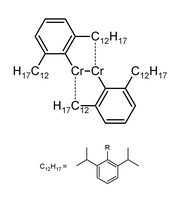
Chromium is notable for its ability to form quintuple covalent bonds. Writing in Science, Tailuan Nguyen, a graduate student working with Philip Power of the University of California, Davis describes the synthesis of a compound of chromium(I) and a hydrocarbon radical which was shown via X-ray diffraction to contain a quintuple bond of length 183.51(4) pm (1.835 angstroms) joining the two central chromium atoms.[1] This was accomplished through the use of an extremely bulky monodentate ligand which through its sheer size prevents further coordination. Chromium currently remains the only element for which quintuple bonds have been observed.
[edit] Applications
Uses of chromium:
- In metallurgy, to impart corrosion resistance and a shiny finish :
- as an alloy constituent, such as in stainless steel in cutlery
- in chrome plating,
- in anodized aluminium, literally turning the surface of aluminium into ruby.
- As dyes and paints :
- Chromium(III) oxide is a metal polish known as green rouge.
- Chromium salts color glass an emerald green.
- Chromium is what makes a ruby red, and therefore is used in producing synthetic rubies.
- also makes a brilliant yellow for painting
- As a catalyst.
- Chromite is used to make molds for the firing of bricks.
- Chromium salts are used in the tanning of leather.
- Potassium dichromate is a chemical reagent, used in cleaning laboratory glassware and as a titrating agent. It is also used as a mordant (i.e., a fixing agent) for dyes in fabric.
- Chromium(IV) oxide (CrO2) is used to manufacture magnetic tape, where its higher coercivity than iron oxide tapes gives better performance.
- In well drilling muds as an anti-corrosive.
- In medicine, as a dietary supplement or slimming aid, usually as chromium (III) chloride or chromium(III) picolinate.
- Chromium hexacarbonyl (Cr(CO)6) is used as a gasoline additive.
- Chromium boride (CrB) is used as a high-temperature electrical conductor.
- Chromium (III) sulfate (Cr2(SO4)3) is used as a green pigment in paints, in ceramic, varnishes and inks as well as in chrome plating.
- Chromium (VI) is used in the post Ballard preparation of Gravure (rotogravure) printing Forme Cylinders. By electroplating the metal onto the second coat of copper (after the Ballard skin), the longevity of the printing cylinder is increased.
[edit] Biological role
Trivalent chromium (Cr(III), or Cr3+) is required in trace amounts for sugar metabolism in humans (Glucose Tolerance Factor) and its deficiency may cause a disease called chromium deficiency. In contrast, hexavalent chromium is very toxic and mutagenic when inhaled as popularized by the film Erin Brockovich. Cr(VI) has not been established as a carcinogen when not inhaled but in solution it is well established as a cause of allergic contact dermatitis (ACD).[2]
Recently it was shown that the popular dietary supplement chromium picolinate complex generates chromosome damage in hamster cells. In the United States the dietary guidelines for daily chromium uptake were lowered from 50-200 µg for an adult to 35 µg (adult male) and to 25 µg (adult female).[3]
[edit] Isotopes
Naturally occurring chromium is composed of three stable isotopes; 52Cr, 53Cr, and 54Cr with 52Cr being the most abundant (83.789% natural abundance). Nineteen radioisotopes have been characterized with the most stable being 50Cr with a half-life of (more than) 1.8x1017 years, and 51Cr with a half-life of 27.7 days. All of the remaining radioactive isotopes have half-lives that are less than 24 hours and the majority of these have half-lives that are less than 1 minute. This element also has 2 meta states.
53Cr is the radiogenic decay product of 53Mn. Chromium isotopic contents are typically combined with manganese isotopic contents and have found application in isotope geology. Mn-Cr isotope ratios reinforce the evidence from 26Al and 107Pd for the early history of the solar system. Variations in 53Cr/52Cr and Mn/Cr ratios from several meteorites indicate an initial 53Mn/55Mn ratio that suggests Mn-Cr isotope systematics must result from in-situ decay of 53Mn in differentiated planetary bodies. Hence 53Cr provides additional evidence for nucleosynthetic processes immediately before coalescence of the solar system.
The isotopes of chromium range in atomic weight from 43 u (43Cr) to 67 u (67Cr). The primary decay mode before the most abundant stable isotope, 52Cr, is electron capture and the primary mode after is beta decay.
[edit] Precautions
Chromium metal and chromium(III) compounds are not usually considered health hazards; chromium is an essential trace mineral.[4] However, hexavalent chromium (chromium VI) compounds can be toxic if orally ingested or inhaled. The lethal dose of poisonous chromium (VI) compounds is about one half teaspoon of material. Most chromium (VI) compounds are irritating to eyes, skin and mucous membranes. Chronic exposure to chromium (VI) compounds can cause permanent eye injury, unless properly treated. Chromium(VI) is an established human carcinogen. An investigation into hexavalent chromium release into drinking water formed the plot of the motion picture Erin Brockovich.
World Health Organization recommended maximum allowable concentration in drinking water for chromium (VI) is 0.05 milligrams per liter. Hexavalent chromium is also one of the substances whose use is restricted by the European Restriction of Hazardous Substances Directive.
As chromium compounds were used in dyes and paints and the tanning of leather, these compounds are often found in soil and groundwater at abandoned industrial site, now needing environmental cleanup and remediation per the treatment of brownfield land. Primer paint containing hexavalent chromium is still widely used for aerospace and automobile refinishing applications.
[edit] See also
[edit] References
- ^ T. Nguyen, A. D. Sutton, M. Brynda, J. C. Fettinger, G. J. Long and P. P. Power (2005). "Synthesis of a Stable Compound with Fivefold Bonding Between Two Chromium(I) Centers". Science 310 (5749): 844-847. DOI:10.1126/science.1116789.
- ^ Template:Cite ref
- ^ Vincent, J.B. (2007). "Recent advances in the nutritional biochemistry of trivalent chromium". Proceedings of the Nutrition Society 63 (01): 41-47. DOI:10.1079/PNS2003315. Retrieved on 2007-07-12.
- ^ Chromium. Wellness Letter.
BW Bewise Inc.
Welcome to BW tool world! We are an experienced tool maker specialized in cutting tools. We focus on what you need and endeavor to research the best cutter to satisfy users’ demand. Our customers involve wide range of industries, like mold & die, aerospace, electronic, machinery, etc. We are professional expert in cutting field. We would like to solve every problem from you. Please feel free to contact us, its our pleasure to serve for you. BW product including: cutting tool、aerospace tool .HSS Cutting tool、Carbide end mills、Carbide cutting tool、NAS Cutting tool、Carbide end mill、Aerospace cutting tool、Фрезеры’Carbide drill、High speed steel、Milling cutter、CVDD(Chemical Vapor Deposition Diamond )’PCBN (Polycrystalline Cubic Boron Nitride) ’Core drill、Tapered end mills、CVD Diamond Tools Inserts’PCD Edge-Beveling Cutter(Golden Finger’PCD V-Cutter’PCD Wood tools’PCD Cutting tools’PCD Circular Saw Blade’PVDD End Mills’diamond tool ‘Single Crystal Diamond ‘Metric end mills、Miniature end mills、Специальные режущие инструменты ‘Пустотелое сверло ‘Pilot reamer、Fraises’Fresas con mango’ PCD (Polycrystalline diamond) ‘Frese’Electronics cutter、Step drill、Metal cutting saw、Double margin drill、Gun barrel、Angle milling cutter、Carbide burrs、Carbide tipped cutter、Chamfering tool、IC card engraving cutter、Side cutter、NAS tool、DIN tool、Special tool、Metal slitting saws、Shell end mills、Side and face milling cutters、Side chip clearance saws、Long end mills、Stub roughing end mills、Dovetail milling cutters、Carbide slot drills、Carbide torus cutters、Angel carbide end mills、Carbide torus cutters、Carbide ball-nosed slot drills、Mould cutter、Tool manufacturer.

No comments:
Post a Comment